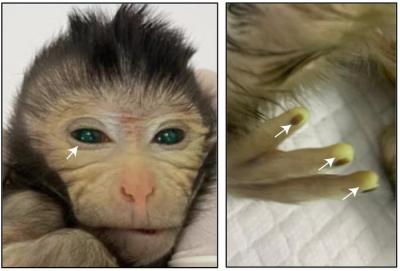
The images are unsettling, to put it mildly: a baby monkey whose skin grows eerily green, with glowing fingertips reminiscent of E.T.
This is “#10,” the world’s first live-born monkey that’s largely made up of embryonic stem cells, or ESCs, from a different embryo—in other words, a chimera. It’s the work of researchers at the Chinese Academy of Sciences in Beijing, who reported its creation Nov. 9 in Cell. While it’s not the first time scientists have facilitated the live birth of chimeric animals, it is the first time they’ve done so in a monkey. The development is both a major advancement for stem cell research and an ethics conundrum.
“Making chimeric monkeys could facilitate new kinds of genetic and developmental biology studies with more direct relevance to humans,” Paul Knoepfler, Ph.D., a stem cell biologist and cancer researcher at the University of California, Davis who was not involved in the study, told Fierce Biotech Research in an email interview. But, he added later, “there are some difficult questions and ethical considerations arising from this new paper.”
The monkey at the heart of the study was the survivor of a long process that began with the scientists removing ESCs from seven-day-old monkey blastocysts and growing them into nine different stem cell lines, using techniques from human pluripotent stem cell studies to do so. The ESCs were placed in a special culture medium to enhance their ability to differentiate into the cell types necessary to grow into a live animal and were labeled with a green fluorescent protein, or GFP, that would allow the scientists to see which tissues had grown from them.
After growing the stem cells, they selected a subset of them and injected them into 206 morulae, four- to five-day old embryos about the size of a mulberry. Of these, 74 of them were ultimately implanted into 40 different surrogate mothers. This led to 12 pregnancies, which ended in four aborted fetuses and six live births.
Only two of the resulting animals—one of the aborted fetuses and one live male monkey, dubbed “#10” in the paper—had significant GFP signals indicating that they were largely chimeric. But #10 was, indeed, “strikingly” green, the researchers wrote: GFP could be seen throughout his body, including in his eyes, fingers and tail, “indicating a high-level contribution of ESC-derived cells.” DNA profiling called short-tandem-repeat analysis confirmed the ESCs' presence.
Unfortunately, #10 didn’t survive long. He developed respiratory failure and hypothermia 10 days after birth, the paper said, and was euthanized by a veterinarian.
But it was clear that the process did ultimately accomplish its goal: In #10, analysis of 26 different tissues showed that ESC contributed to between 21% and 92% of their composition, with an average of 67%. This is much higher than what was seen in the primate fetuses that came from earlier attempts by other research groups to create monkey chimeras, co-author Miguel Esteban, Ph.D., said in a Nov. 7 press conference.
“The thing is that previous studies had solo contribution[s of ESCs]—you cannot really call [the resulting fetuses] chimeric animals,” Esteban said. “They just had several cells sparsely distributed all over the body, with no real structure formation.”
In contrast, the ESCs in the primates from the new study, including #10 and the aborted fetus, formed into complex organs throughout their bodies. On top of that, Estaban noted, the ESCs also incorporated into germ line cells—sex cells that carry DNA from parent to offspring—and into the placenta.
Getting this to work “was extremely challenging at a technical level,” Knoepfler said. “It seems the key advance here was learning from human pluripotent stem cell culture methods to apply them to monkey cells.”
But the study presents ethical quandaries on two different fronts, he added. First, it opens the door to more extensive human-monkey chimeras, embryonic forms of which have already been made. While this could yield “unique and beneficial knowledge,” Knoepfler said (and has previously written about), such work is ethically complex.
“For example, how long is it permissible to grow and study a human-monkey chimera embryo? How does one avoid having extensive human brain cells in such a chimera if you study it for long periods or is that permissible in some cases?” he said. “These questions apply to other types of human-animal chimeras that have been made as well.”
A more prescient concern is the inefficiency of the process to generate live monkey chimeras in the first place, Knoepfler added. Only one of the 74 monkey embryos transferred was born a live chimera—a result that required 40 female monkeys to undergo surgery. And while #10 was born alive, he wasn’t healthy.
““There was likely some suffering involved for that animal, which should be factored into planning for possible future research as an important risk,” Knoepfler said.