 |
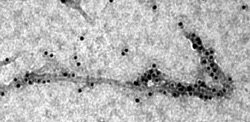
| Transmission electron microscopy image showing a molecular chaperone binding to threadlike amyloid-beta--Courtesy of Samuel Cohen |
One of the chief focuses in Alzheimer's drug research centers on the amyloid beta theory: toxic clusters of the protein form in the brain, triggering a disease that robs a person of his memories. So finding ways to blunt amyloid or flush it from the brain has consumed billions of dollars in R&D cash--so far unsuccessfully. Now a team of scientists at some of the most prestigious research institutions in the U.K. and Sweden say they've found a molecule that could disarm these toxic clusters, offering a new approach to treating the disease.
The molecule is the naturally occurring Brichos, a "chaperone" that sticks to amyloid fibrils and keeps them from connecting, preventing or slowing the onset of damaging clusters that could be behind the disease.
As is typical of much of the research in the field, the scientists begin with the assumption that they're right about amyloid beta being the trigger for the disease. But it's important to note that these clusters also occur in people who don't develop Alzheimer's, leading regulators to take a more cautionary approach when it comes to what causes the disease. Also, late-stage inhibitors of amyloid beta have failed to slow or stop the disease, causing a host of researchers to move upstream to the earliest stages of the disease while others pursue tau and other targets.
Investigators, nevertheless, are hot on the trail of other molecules that can accomplish the same task, searching for new drug targets that can be studied in the clinic. They note that once the proteins start to misfold, amyloid fibrils accelerate the process, hastening the development of the suspect clusters. Preventing that second stage of the disease, they say, could make a major impact on the disease.
 |
| Samuel Cohen |
"A great deal of work in this field has gone into understanding which microscopic processes are important in the development of Alzheimer's disease; now we are now starting to reap the rewards of this hard work," says Samuel Cohen, a research fellow at St John's College, Cambridge, and a lead author of the report. "Our study shows, for the first time, one of these critical processes being specifically inhibited, and reveals that by doing so we can prevent the toxic effects of protein aggregation that are associated with this terrible condition."
Cohen is part of a group drawn from the Department of Chemistry at the University of Cambridge, the Karolinska Institute in Stockholm, Lund University, the Swedish University of Agricultural Sciences and Tallinn University. Their findings are reported in the journal Nature Structural & Molecular Biology.
- here's the release