Roche has a plan to build out its point-of-care diagnostic catalog, with the goal of delivering more tests to homes, pharmacies, doctor’s offices and elsewhere. The company aims to acquire a selection of products from the flagging LumiraDx, including its shoebox-sized analysis instrument and a menu of specialized testing strips.
The deal, expected to take effect by the middle of this year, includes a payment of $295 million upfront. Another cache of up to $55 million has been set aside to reimburse LumiraDx and its backer BioPharma Credit for keeping its point-of-care business afloat until the handover is complete.
Last October, LumiraDx said it received a notice from the Nasdaq that it would be delisted from the stock exchange after its share price lagged below $1 for 30 consecutive days. At the time, the company said it planned to appeal. Its compliance had also been in doubt the previous February.
The London-based developer has also been undergoing a strategic review process since mid-2023, after laying off about 40% of its workforce earlier in the year.
Alongside the deal with Roche, the company announced that it had appointed three joint administrators for its businesses from the firm FTI Consulting. LumiraDx said it anticipates that all of the proceeds from the sale will go toward outstanding loan debts and that no profits would be distributed to the company or its shareholders.
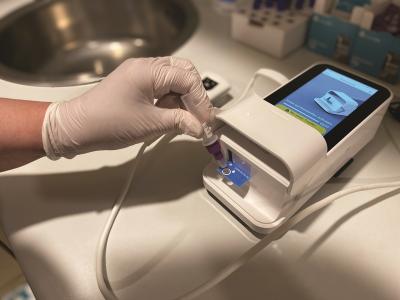
The company’s second-quarter 2023 financial results posted $21 million in revenue, down from $44 million during the same period the year before, including $9.2 million in non-COVID sales, versus nearly a $50 million net loss after expenses. It said it shipped more than 1,500 new instruments during that time, primarily to U.S. pharmacies. Last year, it also submitted its first application to the FDA for a traditional 510(k) clearance, for a five-minute COVID test.
This week, LumiraDx’s share price briefly spiked from about 6 cents to 12 cents at the first disclosure of the deal, but the value has since fallen to about 4 cents as of Jan. 2.
LumiraDx had previously made a name for itself during the early stages of the COVID-19 pandemic by claiming one of the FDA’s earliest emergency authorizations for a point-of-care antigen test.
Early 2021 revenue projections that ranged from $600 million to $1 billion led the company to chart a course to the public markets via a $5 billion SPAC transaction—but within months, steep declines in the demand for COVID tests forced LumiraDx to slash $2 billion off the announced value of its planned reverse merger. Still, its Nasdaq debut in late September of that year made it one of medtech’s biggest M&A deals of 2021.
LumiraDx’s loss appears to be Roche’s gain: The company specifically highlighted how LumiraDx’s immunoassay and clinical chemistry test strips can be stored at room temperature, making them a good fit for Roche’s global affiliate and distribution network.
“We believe this will enable better patient access to timely results in decentralised healthcare settings worldwide,” Roche Diagnostics CEO Matt Sause said in a statement. Roche also said it plans to develop additional tests for the system in the future and build a new complement to its lab-based, high-throughput diagnostics portfolio.