Since the technology’s first FDA clearance a decade ago, stent retrievers have been a go-to tool for the removal of blood clots, including those that block the flow of oxygenated blood to the brain and cause ischemic strokes. After being inserted into an artery and threaded toward the clot, a standard stent retriever will automatically expand around the thrombus before being pulled back out.
But the devices have been linked to a combined failure, complication and death rate as high as 40%, according to some studies, since the retrievers can be difficult to navigate through the tangly vascular system and may not give surgeons much control over the actual clot-capturing process.
That’s where Rapid Medical comes in. The Israeli startup has developed a stent retriever system that allows surgeons to operate the device’s expansion and that, in its latest iteration, is believed to be the smallest thrombectomy device currently on the market.
The sized-down TigerTriever 13 device will now be available to U.S. surgeons, thanks to a newly bestowed FDA clearance, the company announced this week.
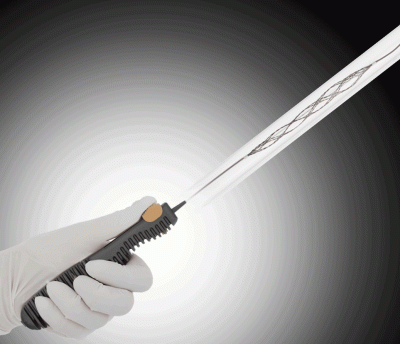
TigerTriever 13 comprises a handheld controller and a microcatheter fitted with a mesh clot-grabbing net that’s only 2.5 millimeters in width when fully expanded. That tiny profile allows it to be used to remove clots from blood vessels as small as 1 millimeter in width. According to Rapid, blockages in vessels ranging from 1 millimeter to 2.5 millimeters may make up as many as 30% of ischemic stroke cases—but, until now, have been unreachable by other stent retrievers.
The device’s remote features a yellow slider that surgeons can use to expand and compress the net. That’s in contrast to standard stent retrievers, which automatically expand once they’ve reached the clot and then have to be pulled back through the artery while still fully expanded.
Being able to compress the device’s net after grabbing hold of a thrombus may help reduce resistance and tension in the vascular system during the removal process, the company said.
FDA clearance for the TigerTriever 13 system comes several years after it was given the go-ahead with CE mark approval in Europe in 2018. Meanwhile, Rapid has racked up a handful of other nods from the U.S. regulator, including 510(k) clearance last year for a larger version of the TigerTriever device.
A clinical trial of the original TigerTriever device showed that it was more successful than other stent retrievers in removing clots from large blood vessels. Results of that study published (PDF) in early 2021, ahead of the system’s FDA clearance, demonstrated a nearly 85% revascularization success rate for Rapid’s system compared to an average of about 74% for other clot removal devices.
Rapid is also zooming ahead in a new trial of the TigerTriever 13 device. The Distals study will track the system’s ability to remove clots from isolated, harder-to-reach regions of the brain. Success in that trial would open up the device to be used on up to 40% more patients experiencing ischemic stroke.