Four years ago, when NeurAxis was still known by its founding name, Innovative Health Solutions, it earned an FDA de novo clearance for the IB-Stim device as a neuromodulation treatment for teenagers experiencing pain associated with irritable bowel syndrome.
Now, the company has published study results proving that the technology can provide pain relief for a broader swath of the class of conditions known as disorders of the gut-brain interaction, or DGBIs. These disorders include IBS, reflux hypersensitivity and functional dyspepsia, among others, and often lead to chronic abdominal pain and increased anxiety and depression—and a reduced quality of life overall.
The study results, published earlier this month in Frontiers in Pain Research, suggest that IB-Stim could turn those symptoms around in the matter of just a few weeks.
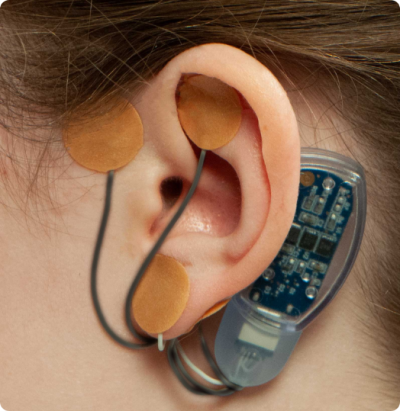
The IB-Stim device is placed behind a patient’s ear. It sends gentle electrical impulses through electrodes to the branches of the peripheral cranial nerve located just under the skin, with an aim of blocking GI-related pain signals on their way to the brain.
The single-arm study, which was conducted at the Children’s Hospital of Orange County in California, recruited 31 patients with pain-related DGBIs. They were between the ages of 11 and 18—with an average age of 15.7 years old—and more than 80% were female.
After undergoing treatment with IB-Stim’s percutaneous electrical nerve field stimulation therapy once a week for four weeks, patients reported “significant reductions” from their baseline scores of abdominal pain, nausea severity, functional disability and somatization—improvements backed by their parents’ own surveys, too.
While the teens’ self-reported quality of life scores didn’t show a similarly statistically significant change over the course of the four weeks, their parents’ ratings did, suggesting they’d observed marked improvements across their kids’ “physical function, psychosocial function and generic core scale scores,” per the study. Additionally, both patients and parents reported significant improvements on the PROMIS Global Health scale, which measures patient-reported outcomes in mental and physical health.
Another measure that didn’t make the statistically significant cut was that of the participants’ self-reported changes in depression symptoms over the course of the study—though the researchers did discover “a significant correlation between decreased abdominal pain intensity and decreased depression.” Meanwhile, anxiety scores did meaningfully improve after four weeks.
“This study is unique in that it not only found improvements in gastrointestinal symptoms, but also in the child’s quality of life based on the parent’s report,” Adrian Miranda, M.D., NeurAxis’ chief medical officer, said in a company announcement Tuesday.
NeurAxis is working to ensure that children have access to necessary and potentially life-improving treatments, Miranda added. To that end, as CEO Brian Carrico noted in the release, the company is using these and other study results to “expand written policy coverage with a goal to drive guideline changes that support IB-Stim as the standard of care for [functional abdominal pain disorders].”