Nearly a year after recalling a crucial component of its Harmony transcatheter pulmonary valve (TPV) system—a process that began barely a year into the device’s initial rollout—Medtronic is striking the right note.
The medtech giant has begun a relaunch of the heart valve, it announced Wednesday, making the Harmony system once again available to U.S. patients who are in need of a pulmonary valve replacement.
“The relaunch of Harmony TPV underscores our continued commitment to advancing solutions for all people who experience heart disease, and we are proud to be driving innovation forward by offering the first non-surgical solution designed for congenital heart disease patients with severe pulmonary valve regurgitation,” Nina Goodheart, president of Medtronic’s structural heart and aortic business, said in the release.
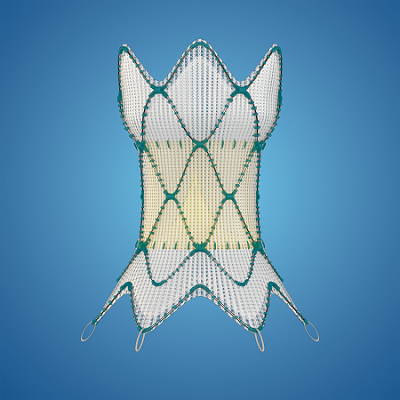
The device is aimed specifically at the approximately 20% of congenital heart disease patients who have their right ventricular outflow tract (RVOT)—which connects the heart and lungs—repaired at birth. According to data cited by Medtronic, the majority of those patients will need a pulmonary valve replacement later in life to treat severe pulmonary valve regurgitation, in which blood that’s been pumped into the lungs leaks back into the right lower chamber of the heart.
When the FDA bestowed its approval in 2021, Harmony TPV was authorized to treat the condition in congenital heart disease patients with RVOT anomalies—making it the first such replacement valve approved for insertion through a minimally invasive, catheter-based procedure, rather than a potentially risky open-heart surgery.
To score the approval, Medtronic submitted the results of a clinical trial in which 90% of the 70 implanted participants met the primary endpoint: a measure of whether any further surgeries or other interventions were needed to correct the valve or a patient’s blood flow within six months of implantation.
But in March 2022, less than a year after landing the regulatory nod, Medtronic began a voluntary recall of the delivery catheter included in the Harmony TPV system. The recall was ultimately given a Class I rating, the FDA’s most serious.
The catheter is the key to the Harmony system’s minimally invasive placement procedure. Medtronic found that the bond that holds the capsule at the end of the delivery catheter may be at risk of breaking during the procedure. If that happens, not only could it delay the implantation while the device is replaced, but it may also require patients to undergo additional surgeries and could cause serious damage to their blood vessels.
As of the FDA’s April recall notice, the catheter issue had sparked six complaints, including one reported injury.
In its own recall notice sent to healthcare providers, Medtronic asked that all unused Harmony TPV delivery catheters be returned to the company and that all planned procedures with the system be put on hold. According to this week’s relaunch announcement, those procedures can now begin again, as the company “worked collaboratively with the FDA to remediate the issue and earn FDA approval to return the device to market.”