Medtronic secured an FDA approval for the use of a new technique in placing its drug-eluting stents, to help treat the build-up of plaques that may clog the junction between two arteries feeding the heart.
Known as bifurcation lesions, these cases can present a special challenge due to differences between the blood vessels, while side branches can be hard-to-reach during a percutaneous coronary intervention. According to the company, these treatments have typically seen fewer successes, and patients have had increased rates of long-term cardiovascular complications.
The agency’s expanded stent indication was handed down to Medtronic’s previously approved Resolute Onyx and its recently launched Onyx Frontier, which received FDA approval in May.
The company said the green light—which covers a provisional technique that uses a single stent to treat the bifurcation—will allow it to provide new medical education and procedural training on treating these difficult lesions.
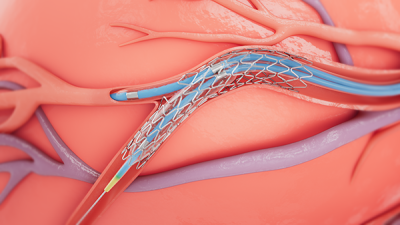
The procedure starts with the deployment of a stent in the larger, main branch of the coronary artery. Then, another guidewire is threaded through the gaps in the struts of the placed stent and into the smaller side branch; this allows a balloon to inflate and press the sides of the scaffolding into the bifurcation, providing structure to both vessels. This can also create an opening for two-stent procedures, where a second implant is deployed into the side branch.
The Onyx Frontier coronary stent—bent into shape from a single wire, with a platinum-iridium core and shell made of cobalt alloy—also received a CE Mark in Europe in August. It slowly delivers a dose of the immunosuppressant zotarolimus to help prevent the blood vessel from reclosing.
This week Medtronic also received a separate CE Mark for a paired stent-graft system designed to help treat complex abdominal aortic aneurysms.
The European approval means the company’s Radiant balloon-expandable covered stent can be used on-label alongside its Endurant II/IIs stent-graft implants, to help tackle chimney endovascular aneurysm repairs, a procedure known as ChEVAR.
In ChEVAR, secondary covered stents are used to route fresh blood to the arteries that branch off the sides of the aorta, feeding the kidneys and other organs, while the larger stent-graft in the central aorta aims to take the pressure off the swelling aneurysm.
The medtech giant said the Radiant covered stent system—developed through a long-term collaboration between Medtronic and Getinge—will launch in Western Europe this November, before expanding to other CE Mark countries in 2023.