Medtronic has secured an FDA green light for a new type of implantable cardioverter-defibrillator that the medtech giant describes as the first of its kind.
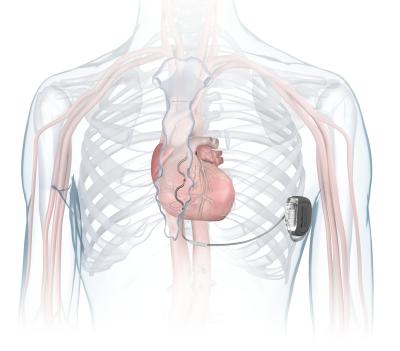
Unlike other ICDs that are wired into beating hearts through the body’s veins, the Aurora’s electrical leads can be placed outside of the cardiac muscle and blood vessels to help reduce the risks of long-term complications.
The Aurora extravascular ICD can deliver defibrillation to patients in cardiac arrest as well as ongoing pacemaker therapies, while maintaining a size, shape and battery life similar to the company’s traditional transvenous ICDs—but instead of being placed under the collarbone, it is slipped beneath the skin under the left armpit. Its Epsila leads are then connected to the heart under the breastbone through a minimally invasive procedure.
“This FDA approval paves the way for patients to have a better overall experience with ICD therapy,” Alan Cheng, chief medical officer of Medtronic’s cardiac rhythm management business, said in a statement.
“ICDs remain the gold standard for prevention of sudden cardiac death, and while the subcutaneous ICD avoids certain complications associated with transvenous defibrillators, it has limitations that may affect a patient's comfort and quality-of-life,” Cheng added.
Some issues associated with transvenous leads have included narrowing, blocking or compressing of the blood vessels, occluding their flow, in addition to the risk of blood infections.
“With the Aurora EV-ICD system, patients can benefit from the only ICD placed outside the vascular space that provides [anti-tachycardia pacing] and back-up pacing, in a device that is nearly half the size and with 60% greater projected battery longevity compared to the competitor's subcutaneous ICD,” Cheng said.
The implant is also equipped with Medtronic’s SureScan technology that allows a patient to undergo an MRI while the device is implanted, plus the company’s Smart Sense algorithm, which aims to reduce the potential for inappropriate shocks.
The Aurora EV-ICD received a CE mark allowing its sale in Europe this past February. The approvals on both sides of the Atlantic also cover proprietary tools for its implantation procedure. The company previously set a European launch date of this autumn and said its initial U.S. commercial release would begin on a limited basis within the coming weeks.
A previous single-arm clinical study of 356 patients clocked the Aurora’s effectiveness at delivering defibrillation at 98.7%. The company said there were no procedural complications, nor any unique complications related to the placement or operation of the extravascular device compared to traditional ICD implants.
Additionally, after six months, 92.6% of patients were free from major system- and procedure-related complications, such as hospitalization, the need for revision surgery or death. The results were published last October in the New England Journal of Medicine.
Medtronic also said that its system was able to avoid delivering 33 shocks during the study by providing anti-tachycardia pacing, allowing it to interrupt dangerously fast rhythms before they cause a potentially fatal stoppage in blood flow.