About eight months after claiming stateside approvals, Medtronic has secured a green light in Europe for the latest generation of its miniaturized leadless pacemakers.
The CE mark covers the company’s Micra AV2 and VR2 cardiac implants, which the medtech giant describes as the world’s smallest. While weighing less than 2 grams, they are designed to offer about 40% more battery life compared to their predecessors, with device lifespans of nearly 16 and 17 years, respectively.
Medtronic has estimated that that amount of time will cover more than 80% of patients who receive pacemakers. About the size of a large vitamin capsule, the Micra AV2 and VR2 are about one-tenth the size of a traditional pacemaker implant, and are embedded directly within the heart’s chambers via a minimally invasive procedure. The system is also capable of reporting data to clinicians remotely.
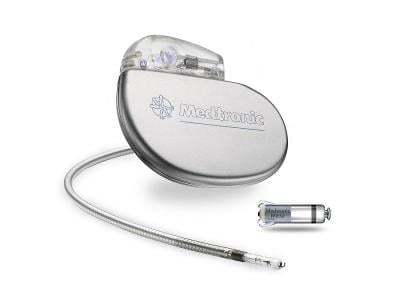
The previous Micra AV was first approved in the U.S. and Europe for dual-chamber use in 2020, with a battery life between eight and 13 years. The single-chamber Micra VR, meanwhile, could last nearly 14 years. The original Micra TPS device first received a CE mark in 2015. Medtronic estimates that more than 200,000 patients worldwide have received a Micra pacemaker.
“For more than eight years, our Micra leadless pacemakers have provided meaningful benefits to people in Europe who require a pacemaker,” Robert Kowal, Medtronic’s general manager of cardiac pacing therapies, said in a statement. “Now, these patients have access to the latest leadless pacing technology that, for most of them, may be the only device they will ever need.”
Last year the FDA also approved competing leadless hardware from Abbott, the Aveir DR, which allows for separate, paired implants to be placed in different places within the heart’s atrium and ventricle while synchronizing their pacing wirelessly. According to Abbott, the dual-chamber system is currently undergoing regulatory review in Europe; a single-chamber Aveir implant received a CE mark in July 2023.