Mere weeks after announcing the first insulin pump partner for its FreeStyle Libre 2 Plus continuous glucose monitor—in which the “Plus” designates an FDA-cleared ability to link up with automated insulin delivery systems—Abbott has found a second such tie-up across the pond.
Last month’s update paved the way for U.S. users to integrate the Libre 2 Plus sensor with Tandem Diabetes Care’s t:slim X2 insulin pump and Control-IQ automated dosing algorithm. Now, according to a Wednesday announcement, Libre 2 Plus users in Europe can construct an artificial pancreas of their own by pairing their Abbott devices with Insulet’s insulin pump and dosing algorithm.
The newly bestowed CE mark allows the combined system to be used by people with Type 1 diabetes who are at least 2 years old. The technology integration will roll out first in the U.K. and the Netherlands throughout the first half of this year, after which it will expand to other countries.
Elsewhere on the continent, Insulet’s Omnipod 5 tech is already compatible with Dexcom’s G-series CGMs—so, according to Insulet, the new approval makes Omnipod 5 the only tubeless hybrid closed-loop system to be CE marked for use with multiple brands’ glucose sensors.
Meanwhile, also in Europe, the Libre 3 model of Abbott’s CGM technology can be used as part of a closed-loop system with Ypsomed’s insulin pump and CamDiab’s algorithm-equipped app.
Abbott’s Libre 2 Plus sensor is placed on the back of the upper arm, where it can stay for up to 15 days. It collects blood sugar readings every minute and automatically transmits them to an accompanying mobile app.
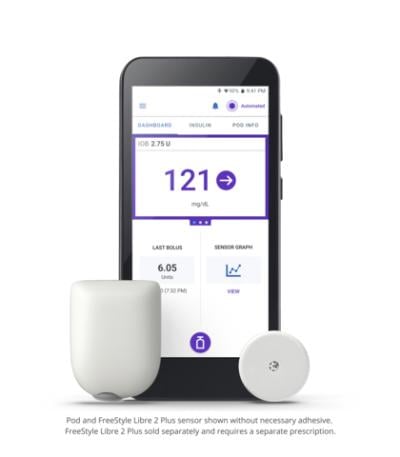
When the CGM is connected to an automated insulin delivery system like Insulet’s, the glucose data is processed by an algorithm that then automatically adjusts an insulin pump’s output as needed throughout the day. Specifically, the Omnipod 5 system’s SmartAdjust technology is able to predict how glucose levels may change up to an hour in advance and makes corresponding adjustments to the insulin pump every five minutes to keep a user within their target glucose ranges.
The aim of these artificial pancreas systems is to reduce the burden on individuals with diabetes to have to constantly monitor their blood sugar levels and insulin dosages.
The collaboration between Abbott and Insulet has been in the works for quite some time. In the announcement about their FDA clearance nearly a year ago, Abbott mentioned that it was already in the process of integrating the Plus versions of the Libre 2 and 3 sensors with both Tandem and Insulet’s automated insulin delivery systems.
A few months later, during the American Diabetes Association’s annual meeting in June, Insulet said it was preparing to launch a study of the combined Omnipod 5 and FreeStyle Libre 2 Plus system. The study, which is still in progress, aimed to recruit up to 200 people of all ages with Type 1 diabetes in the U.K., France and Belgium.
In Wednesday’s announcement, Patrick Crannell, a senior VP at Insulet and the company’s international general manager, called the new CE mark a “key milestone,” noting that expanding the accessibility of Omnipod 5 “has been a priority for Insulet since the system launched in 2022.”