No more squeezing: The FDA has cleared a smartwatch-like blood pressure device that foregoes the need for an inflatable cuff.
Developed by LiveMetric, the electronic wearable can take a reading every 10 seconds, using an array of tiny sensors that can physically measure the minuscule movements of blood as it passes under the skin beat by beat.
“The LiveOne device is intended to combat the worldwide epidemic of hypertension by offering patients and providers meaningful, deeply personalized health information so action can be taken in real-time,” LiveMetric VP Kelly Benning said in a statement.
“With the same ease as wearing a watch, the LiveOne device will change how hypertension and cardiovascular disease are managed and treated by offering people a thorough understanding of how their lifestyle, behavior, and medication impact their blood pressure,” Benning said.
The device can be worn day or night, allowing for 24-hour logs of changes in blood pressure, by tracking the wrist’s radial artery without requiring separate calibrations.
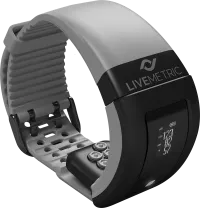
LiveMetric said it would first make the device and monitoring service—which has also received a CE mark in Europe—available through health systems and insurers to people with cardiovascular disease and high blood pressure. It is intended to be used by adults age 27 and older in a clinical setting, according to the FDA.
Earlier this year, the company published the results of a study in the American Journal of Hypertension comparing the LiveOne device to simultaneous blood pressure measurements gathered in-hospital through an invasive arterial line.
Among 34 patients, 91% of systolic blood pressure measurements fell within 10 mmHg of each other, along with 92% of diastolic pressure readings.
Other companies have been pursuing light-based, as opposed to physical, sensors for tracking blood pressure. Rockley Photonics, which has partnered up with Apple, has been developing sensors for a variety of biomarkers—including blood sugar levels, blood alcohol content and hydration—that could one day end up in the tech giant’s smartwatches.
In January, Rockley reported early successes in a pilot study of its wrist-worn, laser-powered blood pressure sensors, setting the stage for broader experiments.
And last September, the FDA cleared a wireless system designed to track a patient’s blood pressure while they’re undergoing surgery, with a sensor that attaches to the finger. Caretaker Medical’s VitalStream device measures the pressure of each heartbeat to deliver ICU-grade vital signs.
More recently, Biobeat—which previously received a blood pressure clearance for its smartwatch and chest patch in 2019—added additional monitoring features to its wearable sensors, with agency green lights for tracking respiratory rate and body temperature.