An outside advisory panel convened by the FDA recommended the agency approve Abbott’s transcatheter heart implant for treating a leaky tricuspid valve, saying the TriClip device’s benefits outweighed its risks.
Built on the design of the company’s top-selling MitraClip for mitral valve regurgitation, the TriClip G4 aims to repair the trickier valve on the opposite side of the heart by grasping the valve’s leaflets and holding them together to help stop blood from flowing backward from the right ventricle into the atrium.
“Tricuspid regurgitation can put added strain on the heart and lead to other cardiovascular issues, which can significantly worsen a person's quality of life, but historically there have been few treatment options,” Lars Søndergaard, chief medical officer and VP of medical affairs for Abbott's structural heart division, said in a statement.
“Abbott recognized the unmet need for people with this condition and explored the use of our proven clip-based technology to find a truly life-changing intervention,” Søndergaard added. “TriClip offers an urgently needed alternative that is safe and effective for people who require tricuspid valve repair but are not able to withstand surgery.”
The FDA’s advisory committee meeting this week follows the agency’s approval earlier this month of Edwards Lifesciences’ Evoque transcatheter implant designed to fully replace a failing tricuspid valve—which marked the first tricuspid-specific device treatment to obtain a U.S. approval.
Both the Evoque and the TriClip have received green lights in Europe, with the latter’s latest version obtaining a CE mark in April 2021. According to Abbott, the TriClip has been approved in more than 50 countries and has treated more than 10,000 patients.
Following a review of the company’s clinical data—including the TRILUMINATE randomized pivotal trial—the FDA’s circulatory system devices panel voted in favor of TriClip on three separate questions, aimed at patients whose tricuspid regurgitation has persisted despite optimal medical therapies.
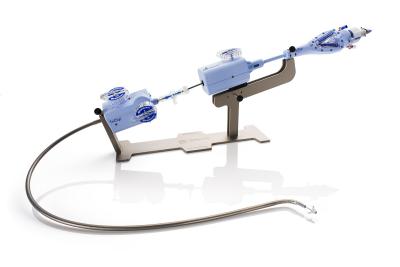
The 14 members voted unanimously that the implant was shown to be safe, while 12 voted yes and two voted no as to whether it also demonstrated it was effective. Finally, on if the TriClip’s benefits outweighed the risks, 13 voted yes and one voted no.
“I just felt the need to pull back a bit on the unbridled enthusiasm,” said panel member Paul Hauptman, dean of the school of medicine at the University of Nevada, Reno, in explaining his vote against.
“I think we have a responsibility to better understand who is going to benefit, and that needs a better definition,” said Hauptman, who also voted against the device’s effectiveness, pointing to how the TriClip’s proposed indication, as it was written, does not make a distinction among patients who may have significant left ventricular dysfunction and low ejection fraction.
Abbott’s TRILUMINATE trial met its primary endpoint, showing superiority against a control group in a composite measurement of death, hospitalizations and repeated tricuspid surgeries—as well as improvements in patient-reported outcome scores using the Kansas City Cardiomyopathy Questionnaire, or KCCQ, a self-administered test that logs symptoms, physical and social limitations and quality-of-life measures.
However, in their briefing document (PDF) delivered to the advisory committee, FDA reviewers said that the KCCQ results in TRILUMINATE’s unblinded study could be subject to a placebo effect—and that the trial’s primary endpoint success was driven only by the patient-reported outcome scores, as death and subsequent surgery rates were similar between both groups.
“So at the end of the day I ended up being the sole dissenting vote on question number three,” said Hauptman. “But the sponsor did a wonderful job presenting the data. This is a step forward in the sense that—if we’re going to rely on the KCCQ—that this is truly important to patients. We want them to feel better.”
According to Abbott, patients who received a TriClip implant reported sustained quality-of-life improvements through at least one year, while 90% saw reductions in the clinical severity of their tricuspid regurgitation.
Other concerns voiced by panel members included how a clipped valve may or may not interfere with the threading of catheters during future minimally invasive procedures.
“It will be interesting to see, if it becomes available, how structural heart clinicians will make decisions on whether to use an Evoque valve or a TriClip, and in which patients,” said Ralph Brindis, a clinical professor of medicine at the University of California, San Francisco, who voted yes on all three counts. “I think it will also be very important to see how patients will be managed afterward with TriClip—including any needs for pacemakers, or so forth—or even if an Evoque valve will become necessary in the future.”