Theranos had a lot of bad ideas, but making blood draws smaller and easier wasn’t necessarily one of them. A decade after the now-defunct blood-testing startup emerged from stealth, BD has now claimed an FDA clearance for fingerstick collection hardware that aims to bring the most common blood tests to pharmacies, grocery stores and other retail locations.
The green light marks a major milestone in the blood collection giant’s long-running collaboration with Babson Diagnostics, the Fierce Medtech Fierce 15 winner that’s been co-developing an effective capillary blood draw system with BD since 2016.
The fruit of their labors is the BD MiniDraw. The device only needs about one-tenth of the volume compared to a traditional blood sample siphoned from a vein, and it addresses what Babson describes as “the typical pitfalls of capillary collection”—namely, surface contamination and how the physical pressure of squeezing out the blood can ultimately hinder a test’s accuracy.
The FDA’s 510(k) clearance covers tests used to help diagnose conditions such as high cholesterol and hypertension, including lipid panels, chemistry assays and hemoglobin and hematocrit levels. BD said it plans to expand the MiniDraw to more uses in the future.
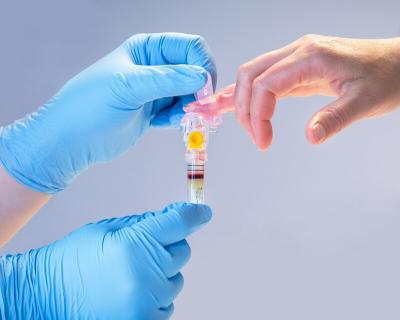
The process needs the hands of a trained healthcare provider but does not require a specialized phlebotomist—making it an option for doctor’s offices as well as other locations, such as urgent care centers and skilled nursing facilities.
“Because the BD MiniDraw Collection System enables blood collection at non-traditional sites that may be more convenient—like your local pharmacy rather than a standalone lab—we can expand health equity and access, and make it easier for patients to get the blood tests they need both for preventative care and the management of chronic conditions,” said BD’s life sciences president, Dave Hickey.
MiniDraw will also serve as the cornerstone of Babson’s upcoming BetterWay blood testing service for primary care and family medicine, slated to launch in 2024.
Using its pea-sized drops of blood, the company aims to offer a slate of tests including liver and kidney panels, as well as complete blood cell counts and more, all through its own automated laboratory.
“Blood testing has remained largely unchanged over the past 70 years, yet it is a critical component of the care journey,” said Babson CEO David Stein. “BetterWay will help address the ongoing shortage of healthcare professionals as well as the increasing need to expand access and reduce healthcare costs. Our mission is to deliver a better experience at convenient locations, with easy-to-understand, medically accurate results trusted by clinicians.”