Two years after its launch as a joint venture between the Mayo Clinic and Nference to develop artificial intelligence algorithms capable of pulling out new insights from routine electrocardiogram results, Anumana has earned its first 510(k) clearance from the FDA for one of those tools.
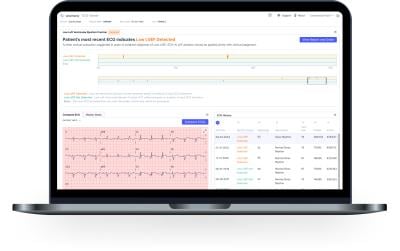
The ECG-AI LEF algorithm was crafted to analyze the peaks and valleys of a 12-lead ECG scan to spot the signs of low ejection fraction in patients with a heightened risk of heart failure. Ejection fraction measures the heart’s ability to effectively pump blood throughout the body, so a lower measurement can indicate the presence of heart failure, potentially leading to cardiac arrest or other life-threatening complications.
Low ejection fraction is typically diagnosed using ultrasound, but Anumana’s tool aims to replace that approach with an automated analysis of faster, simpler, and less expensive ECG scans. Other diagnostic tools include CT, MRI and nuclear medicine scans and a more invasive approach known as cardiac catheterization.
“Anumana’s ECG-AI LEF fills an important unmet need—the lack of an easily accessible point-of-care, noninvasive, and inexpensive tool to screen for a weak heart pump,” Paul Friedman, M.D., chair of the Mayo Clinic’s cardiovascular medicine department and chair of Anumana’s board of advisors, said in a company announcement this week. “It allows identification of otherwise hidden disease, for which many effective, lifesaving treatments are available—once the presence of the disease is known.”
The ECG-AI LEF algorithm was originally developed at the Mayo Clinic, using paired ECG and echo data from tens of thousands of patients.
It has since been put to the test in more than two dozen studies, including a retrospective analysis of 16,000 racially diverse patients in which the AI was able to detect instances of low ejection fraction with nearly 85% sensitivity and weed out negative cases with about 84% specificity, according to Anumana.
Separately, in a clinical trial that spanned more than 22,000 adults receiving routine clinical care at 45 clinics and hospitals, investigators concluded that using the algorithm helped improve clinicians’ ability to accurately diagnose low ejection fraction by 31% compared to the standard of care, with no increase in echo usage. The study’s researchers noted that the AI had been able to find additional cases of low ejection fraction that may have otherwise gone overlooked, using only ECG data already sitting in patients’ electronic health records.
Though the 510(k) clearance announced this week occurred on Anumana’s watch, the ECG-AI LEF algorithm’s history with the FDA dates back well before the Fierce 15 honoree was even established. For one, in 2019, while still fully under the Mayo Clinic’s purview, it earned breakthrough device clearance from the agency, clearing away some obstacles on its path to full clearance.
Then, in the early months of the COVID-19 pandemic in 2020, while the algorithm was licensed to smart stethoscope maker Eko Health, it scored an emergency use authorization from the FDA, enabling it to help spot signs of a weakening heart pump in patients hospitalized with COVID, which can exacerbate cardiovascular issues.