Nestlé Health Science (NHSc) announced a milestone-based acquisition of Phagenesis, a medical device company working on a treatment for dysphagia.
Dysphagia is the inability to swallow safely, and about 29% to 55% of stroke patients experience this condition. It can also be a result of many other diseases.
In this new agreement, NHSc will make an upfront payment to acquire Phagenesis, and will follow with milestone-based funding throughout the clinical evaluation of Phagenyx. Phagenyx is a medical device aimed at treating the cause of dysphagia, rather than only the symptoms. The device helps to restore the neurological control of swallowing.
Using Pharyngeal Electrical Stimulation (PES), Phagenyx aims to treat the neurological cause of dysphagia.
Phagenyx uses a base station and catheter to deliver stimulation to the pharynx. Treatment occurs for 10 minutes a day over three days. Stimulation levels are personalized and can be controlled via the base station.
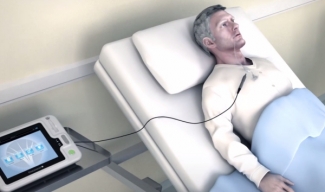
“Nestlé Health Science’s investment positions the company well to address this immense unmet medical need,” Oern Stuge, chairman of Phagenesis, said in the announcement.
“Dysphagia is a strategic focus for Nestlé Health Science,” Greg Behar, CEO of NHSc, said in the announcement. “This innovation can bring a new dimension to swallowing rehabilitation that can be transformational from a patient and healthcare professional perspective.”