--Courtesy of Cefaly Technology
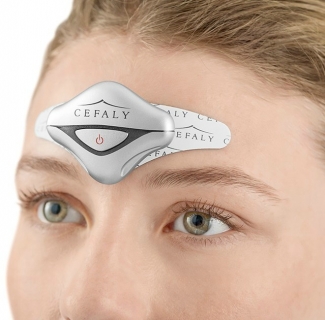
Cefaly Technology has given a serious facelift to its namesake device. The Cefaly I--an external trigeminal nerve stimulation device which helps to prevent episodic migraine attacks--has been shrunk down to three-quarters its size.
The new device--aptly named Cefaly II--can now fit in your palm and and is rechargeable. It is held in place by magnets and offers more control over the intensity of the daily session. It is currently available only in the U.S. but will make its way to Europe in September.
To use the Cefaly II, patients attache a self-adhesive electrode to their forehead, just as they would with the Cefaly I. They then attach the device using a magnetic connection. The use of a magnetic connection means the device will fall into place more quickly and is more secure, reducing the risk of an interrupted session due to any number of small movements.
The device sends electrical impulses through the skin, Cefaly Technology explained. These pulses “desensitize the upper branches of the trigeminal nerve and reduce the frequency of migraine attacks.”
Patients can also control the intensity of the impulses during their session and will have some added convenience in the form of a rechargeable battery.
"This compact device is so easy to tuck in a pocket or purse and I am hopeful it will further increase compliance and bring an even larger reduction in migraine attacks to patients," said Pierre Rigaux, CEO of Cefaly Technology. He also noted that in clinical trials, 81% of compliant patients experienced a significant reduction in migraine attacks and took migraine medication up to 75% less.
Cefaly also noted that only 4.3% of patients in clinical trials reported side effects, which were all minor and reversible.
- here's the release