Spirometrix has received a CE mark for its Fenom Pro Point-of-Care Breathalyzer.
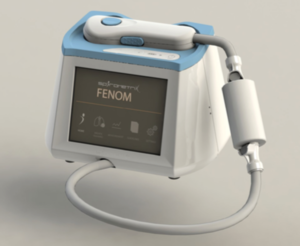
The measurement of the fraction of exhaled nitric oxide (FeNO) has become an established biomarker of asthma. Spirometrix was founded in 2011 by Solomon Ssenyange and Ryan Leard as part of a personal mission to help those suffering with asthma to manage their condition. The company now focuses on developing and commercializing breath analysis devices to be used when diagnosing and managing asthma. The Fenom Pro is one such device.
“FeNO monitoring in the management of asthma patients at the point of care has the potential to optimize asthma therapy, improve compliance of medication use and decrease the need for emergency visits and hospitalization,” James Wolfe, clinical professor of medicine at Stanford University and a scientific advisor to Spirometrix, said in the announcement.
To use the device, a patient breathes into it for about 10 seconds. A reading of nitric oxide levels is available within 30 seconds. At that point the device sends the information to a cloud-based “decision repository” and support system. That system uses big data to find insights about the over 250 million people who suffer with asthma.
For the first time, the Spirometrix respiratory digital ecosystem also includes data from other devices and from the environment. This includes pollen count, local pollution index and air quality. All of these measurements help boost the algorithm which leads to “pattern recognition and pattern management in asthma,” the company explained in the announcement.
The device now has a CE mark and clinical trials for FDA approval will commence this fall.
“The entire Spirometrix team has been working very diligently to achieve this significant milestone, and we believe that receiving CE mark will maintain our strong momentum toward commercialization,” said J. Dean Zikria, CEO of Spirometrix, in the announcement. “The Fenom PRO Point-of-Care Breathalyzer is the first-of-its-kind product in asthma that embraces several sensors and a digital ecosystem designed to effect documentation that may lead to enhanced clinical decision support.”