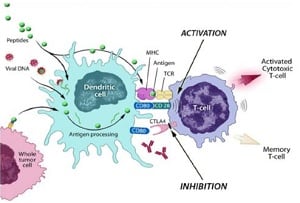
 |
| Provectus' PV-10 mechanism--Courtesy of Provectus |
On Friday afternoon Provectus Biopharmaceuticals spread the word that the FDA had dissed its tumor drug PV-10, refusing to provide a breakthrough therapy designation based on the data it had seen. And Tuesday morning, as its stock ($PVCT) resumed trading, investors inflicted a drubbing on the stock, sending shares down by 80%.
Unlike quite a few biotechs, which wouldn't breathe a word about a rejection like this, Provectus included the FDA's reasoning. "The preliminary clinical evidence you submitted does not indicate that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints," regulators wrote. "Therefore, designation as a Breakthrough Therapy cannot be granted at this time."
CEO Craig Dees, though, touted the rejection, saying the move will help them pursue further development of the therapy. But some analysts, in particular Adam Feuerstein at TheStreet, were caustic in their review.
Feuerstein noted that the FDA letter highlighted the fact that regulators have been demanding a new clinical study of PV-10 for years.
"Provectus and the FDA have been meeting over the same PV-10 phase II data FOR FOUR YEARS!" declared Feuerstein. "The FDA has already told Provectus repeatedly: If you want to compile the data necessary to file PV-10 for approval, please run a phase III study. But Provectus is incapable or unwilling to listen (to) the FDA's advice."
"We are carefully weighing the advice of the Agency and will provide an outline of the path forward for melanoma in our poster presentation June 2 at ASCO," Dees said in his statement on Friday. "We continue to enroll our currently active protocols examining the effect of PV-10 on tumors of the liver; providing expanded access treatment of cutaneous and subcutaneous tumors; and probing the immunologic mechanism of action resulting from tumor ablation with PV-10. With adequate available capital we are in a strong position to execute the necessary steps to bring PV-10 to market for solid tumors and continue development of PH-10 for dermatologic indications."
- here's the release
- here's the review from TheStreet