As a graduate student at the University of California, Los Angeles, Negin Majedi, Ph.D., developed an implantable, spongelike device that may treat solid tumors with fewer side effects than other immunotherapies. Thanks to a new award, its efficacy will be studied in space.
Majedi is the CEO of Symphony Biosciences, a startup built around SymphNode, the immunotherapy device she invented. Her company is one of two new recipients of the Technology in Space Prize, an award sponsored by airline giant Boeing and the Center for the Advancement of Science in Space, the operator of the International Space Station. Symphony and a second startup, FluxWorks, will together receive more than $630,000 total to carry out experiments aboard the ISS—the kind of “once-in-a-lifetime opportunity you tell your grandchildren about,” Majedi told Fierce Biotech Research.
Majedi took part in a Q&A session with Fierce Biotech Research to talk more about SymphNode and how sending it to space will help realize Symphony’s clinical ambitions. The transcript has been edited for brevity.
Fierce Biotech: Congratulations on being awarded the Technology in Space Prize! Let’s start with the basics. What is SymphNode, and how does it work?
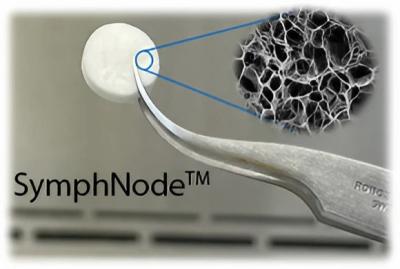
Negin Majedi: SymphNode is a biodegradable device that structurally and functionally mimics the lymph nodes. It is made from an FDA-approved, natural, spongelike material that comes from a seaweed called alginate and can be coated with antibodies or loaded with immune-activating drugs. It’s placed next to solid tumors, where it recruits and activates the patient’s T cells to fight their cancer.

FB: What problem does SymphNode solve?
NM: The goal of our company is to treat solid tumors, such as brain and breast cancer, that are buried deep in the tissues. These types of tumors are harder to treat than liquid cancers, like blood cancer. The treatments that are being used for solid tumors at the moment, such as chemotherapy, are systemic, meaning they bombard the body with all sorts of toxins. This might be okay for blood cancers, but when it comes to solid tumors, the therapeutic outcome is outweighed by the number of side effects. On top of that, when the drugs are stopped, there is a risk that the tumor will grow back or spread to other parts of the body. We think this can be solved with a therapy that is 100% local and works by a better approach that trains the body’s own immune cells to kill the tumor.
FB: What advantages does SymphNode offer over CAR-T therapy?
NM: CAR-T has had really promising results, because it’s the smarter approach where you’re using immune cells to treat cancer rather than relying on drugs. But the treatment process takes a long time, because you first have to isolate the T cells from the patients then go through a procedure of engineering those cells and expanding them. This normally takes four to six weeks, and that time is a luxury that many cancer patients don’t have. Our structure recruits and trains those immune cells on the spot, so the treatment starts pretty much the minute that the diagnosis happens. If an immediate surgery is part of the standard of care, the device can be placed during tumor resection. If surgery is not the first treatment, then we’ll inject SymphNode beside the solid tumor.
FB: Could SymphNode ever be used in conjunction with CAR-T therapy?
NM: This is a standalone treatment that works on its own. But because the device is porous, and because CAR-T therapy has shown great promise on several occasions for some specific types of cancers, we can offer a structure that can be preloaded with CAR-T cells and then be placed beside a solid tumor. In that case, 100% of those engineered T cells would get the chance to reach the tumor, rather than circulating throughout the body and potentially causing side effects like autoimmunity.
FB: Why is it useful to test this technology on the International Space Station?
NM: On Earth, you need a relevant animal model or in vitro model to test your therapy prior to starting clinical trials in humans. But for in vitro models, there are several limitations on Earth that make it hard to recreate a 3D structure of a tumor, because the way those tumors grow in the models is not the way they grow in the human body. That makes it more challenging to study, which is why after the in vitro step, you use the most relevant in vivo models you can find—genetically modified mice, or larger animals. Again, those aren’t 100% realistic because of genetic engineering we must do. Even when you inject them with tumor cell lines, the differentiation of the tumor into different types isn’t even close to 100% similar to what you would see in humans.
But reports on previous work done in space show that the way tumors grow in microgravity gives you a much more realistic way to study how tumor cells actually differentiate into different populations—for instance, a population that stays stationary, like a local tumor, and a population that would start to migrate to other parts. So it’s a great way to study metastasis. That is something you can barely recreate on Earth, whether in mouse models or in vitro.
FB: What kinds of experiments will you be running?
NM: First we will just do a blank control study that will test tumor growth and suppression of the immune cells by the tumor, without any treatment. That will be our baseline. The next step will be to try to replicate the in vitro studies we do on Earth with human T cells and human tumor cells. We will look at the efficacy of immune cells that were trained in the sponges. From this we’ll get a simple fluorescence readout of the tumor death, something you can analyze by microscope, and the immune cells will be frozen and sent back to Earth. Then we’ll do more in-detail RNA sequencing tests and other types of analysis that can give us deeper information about the genetics of those immune cells and the differentiation processes they have been through.
FB: What are some of the challenges that come with running experiments on the ISS?
NM: There are some limitations around the time the astronauts can spend tending to the experiments. Their time is very valuable. So we have to design the experiments in a way that takes the minimum amount of time from the astronauts but gets us the maximum data we can possibly get. We also work with implementation partners on board the ISS who actually have the facility and everything you need to ship experiments to space. A huge portion of our preparation time also has to be spent on figuring out how to match the experimental designs with whatever the implementation partners already have in place in a way that gets optimum results out of this whole effort.
FB: How long will it be before you send the experiments up, and for how long will they be there?
NM: It could take maybe a year or two before we actually launch this into space. The whole experiment will last around two weeks. Then once the spaceship is back, we will have the results and start analyzing them.
FB: What is your timeline for getting your device to clinical trials?
NM: I think it will be two years or so from now until we can actually start our human clinical trials. We are testing SymphNode in larger animal models, such as dogs, and working on figuring out the manufacturing logistics. Efficacy is just one aspect. Another is manufacturing these materials on a larger scale prior to testing them in humans so you can make sure you would have enough supplies.
These efforts and the experiments on the ISS are going to move forward in a parallel way. The results from space will be the cherry on top that will give us the ultimate readout of the therapeutic outcome, as we’ll have already spent the prior two years studying safety and manufacturing. This would be a perfect package for the FDA to give us the green light for starting our human clinical trial.