The FDA is elevating a recall of certain Philips imaging systems, due to a risk of the scanner’s hardware possibly falling onto the patient after the failure of one of its components. The agency handed down a Class I label, its most serious designation, covering Philips’ BrightView line of SPECT/CT imagers.
According to the company, the potential failure of the leadscrew that helps adjust the position of the system’s suspended detector may cause the equipment to move down, toward the patient.
The FDA said that if the detector was positioned below the center of the scanner’s gantry at the time, it could land on the patient’s lower limbs, causing bruising and potential fractures.
If the detector was set above the gantry’s center, its movement would still interrupt the system’s normal operation, potentially requiring the patient to undergo the nuclear medicine procedure from the beginning, with the re-injection of a radiopharmaceutical tracer.
In a statement to Fierce Medtech, Philips said that it had received only one related complaint last year before it issued a field safety notice and began contacting customers in mid-December. The company said there was no report of patient injury or harm.
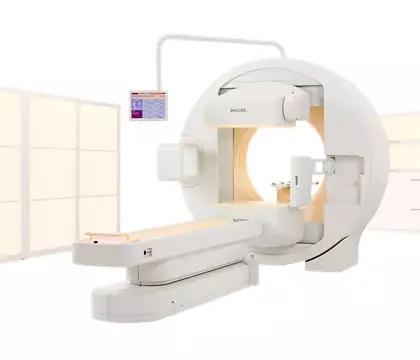
Philips stopped producing the BrightView line in 2014. The notice includes the BrightView, BrightView X and BrightView XCT scanners; about 1,000 systems are still in use worldwide, according to the company, with about 460 of those located within the U.S.
The scanners can still be used, with healthcare providers instructed not to position a patient’s legs directly under the detector below the center of the gantry bore. Philips said in its notice that it would contact customers to schedule an engineer visit to inspect the system, replace the part and add new safety hardware if necessary.
The FDA delivered a Class I recall notice in February 2023 to a similar problem affecting GE HealthCare’s nuclear medicine scanners. About 1,800 of the company’s NM 600 and 800 series machines were found to carry a risk of falling detectors following the failure of a ball screw and a missing safety key—in this case with hardware weighing more than 1,200 pounds.
Philips’ imaging division, meanwhile, collected a Class I recall for its MRI systems last December after an urgent medical device correction detailed the risks of potential explosions following a magnet quench and the release of compressed helium. An unknown blockage in the gas venting system could cause pressure to build up and compromise the structural integrity of the scanner, the company said.