The latest generation of Medtronic’s transcatheter aortic valve replacement implant has passed the FDA’s muster.
Like those that came before it, the new Evolut FX+ is placed through a minimally invasive procedure to treat a narrowed heart valve that severely hampers blood flow to help alleviate chest pain and shortness of breath, among other symptoms.
The main difference in its design lies in the structure of its stentlike skeleton: the addition of larger gaps between struts that would make it easier for a patient to undergo cardiac procedures in the future.
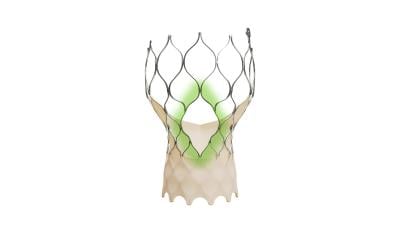
The windows in the self-expanding implant’s frame aim to provide simpler access to catheters bound for the heart’s coronary arteries. Medtronic says the modifications offer openings four times larger than previous Evolut TAVR implants.
“The Evolut FX+ TAVR system was designed to facilitate coronary access across a diverse range of patient anatomies with no compromise to valve performance,” said Jeffrey Popma, M.D., chief medical officer for Medtronic’s coronary, aortic and structural heart businesses, in a company statement.
The FDA’s approval includes patients with severe aortic stenosis across all risk categories for undergoing open heart surgery, spanning extreme, high, intermediate and low. Medtronic said it is planning a limited commercial rollout this spring, with a full product launch set for this summer.