As Americans flock to nearby orchards for festive bouts of autumn apple picking, Insulet is celebrating a particularly bountiful stateside Apple harvest itself.
The diabetes devicemaker has earned FDA clearance for the iPhone version of an app allowing users to control their Omnipod 5 insulin pumps from their own smartphones. Meanwhile, the app has been available to Android owners since the pump’s full U.S. launch began a year ago.
In Insulet’s Monday announcement about the Apple clearance, Eric Benjamin, the company’s chief product and customer experience officer, hailed the impending launch of the app as a “significant milestone in our ongoing effort to provide people with diabetes solutions that improve their lives and help them think less about diabetes.”
The app acts as an alternative to the Omnipod 5 controller—which comes free with a user’s first prescription to the insulin pump—allowing them to avoid carrying around an extra device to manage the pump.
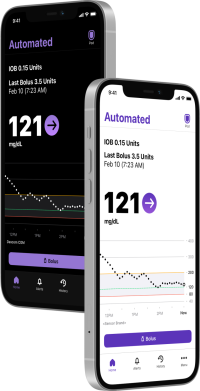
From the app, Omnipod 5 users can perform the initial setup of the system, configure new insulin Pods, adjust their insulin delivery settings and administer and cancel bolus doses of insulin. They can also monitor their pump’s automated insulin delivery, which kicks in once the Omnipod 5 device is linked to a continuous glucose monitor, creating a closed-loop diabetes management system.
The iOS version of the app also boasts an exclusive new feature: dubbed Custom Foods, it aims to simplify mealtime carbohydrate calculations by storing the carb counts for users’ favorite and oft-eaten foods, which can then be added directly to the system’s SmartBolus calculator to make mealtime dosing easier.
To start, the iPhone app will be available only to Omnipod 5 users whose devices are connected to Dexcom G6 CGMs, with the full market release beginning next year. Once it’s available, they’ll be able to download it for free from the Apple App Store and begin connecting it to their existing Omnipod 5 system.
Insulet is still in the process of adapting the Omnipod 5 for use with Dexcom’s latest glucose sensor, the G7.
Insulet claimed in this week’s announcement that the 510(k) clearance makes it the first company to offer a tubeless automated insulin delivery system that can be fully controlled by either an Android or Apple smartphone.
Indeed, while the latest insulin pumps from competitors like Medtronic and Tandem Diabetes Care can connect to their own smartphone apps, they still require infusion set tubing—though both companies have recently moved to acquire companies making tubeless patch pumps, too.
Editor's note: This story was updated to clarify the details of the app's commercial rollout.