 |
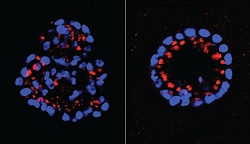
| Mammary epithelial cells with knocked-down expression of PTPD2 resume normal shape (right).--Courtesy of Tonks Lab |
Herceptin was one of the pioneering "personalized" cancer therapies, extending the lives of HER2-positive breast cancer patients. But it also has limitations. Over time, patients tend to develop a resistance to the therapy and the disease can come back and kill them. But investigators at Cold Spring Harbor Laboratory believe that a cocktail approach might take breast cancer treatments to a whole new level, and they've found a new pathway with two promising targets that could help drug developers.
The team of investigators says they believe that they found a new approach to treating HER2-positive breast cancer in a class of enzymes called protein tyrosine phosphatases, or PTPs, which plays a role in cell proliferation.
Using a 3-D model, the group "knocked down" each of the 37 PTPs in the pathway. And eliminating ine of them--PTPD2--stopped cells from developing abnormally, while going on to find an "interaction partner," a lipid called phosphatidic acid (or PA), that could also prove effective in the pathway.
"In this work, Mathangi Ramesh has found a new pathway--a signaling pathway downstream of HER2 that we didn't know about before," CSHL professor Nicholas Tonks says. "Two components of the pathway, the phosphatase PTPD2 and the lipid PA, are together required for HER2 signaling to function in mammary epithelial cells."
"If you can use combination approaches, hitting multiple targets within the cell to reduce the activity of each, and you see a synergistic effect between them, you may be able to overcome some of their harmful effects in HER2-positive cancer, and perhaps also resistance," adds Tonks. "That is our goal."
- here's the release