 |
| Dr. James Bradner |
By Oliver Worsley
Dr. James Bradner and his team at the prestigious Dana-Farber Cancer Institute have recently highlighted a new and sophisticated way to beat cancer.
Rather than adopting the conventional approach of inhibiting proteins and their signalling pathways needed for the survival of the cancerous cell, Bradner's team has been able to tag these proteins for complete degradation by harnessing the cell's natural recycling system. Bradner's lab driving the preclinical experiments dubbed this new technology "degronimids"--relating to the degrading potential of this new class of drugs.
To do this they employed a protein-degrading enzyme called Cereblon. Well known in the anti-cancer field--this endogenous enzyme forms part of the ubiquitin E3 ligase complex--Cereblon tags proteins in the cell for destruction.
By creating an adaptor made from phthalimide--a synthetic analog of the widely used adjunctive cancer drug thalidomide--they designed it to contain a Cereblon-binding pocket as well as being conjugated itself to a BRD4 inhibiting drug, JQ1. The importance of inhibiting the BET-family member, BRD4, has previously been shown by Bradner's group and the original Nature review on BET-inhibition can be read here.
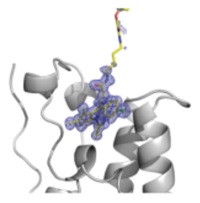 |
| Crystal structure showing the conjugated JQ1-phthalimide, dBET1, bound to the target BET-family member, BRD4--Courtesy of Dana-Farber |
"The potency, selectivity, and rapidity of this approach--namely the ability to home in specifically on BRD4--are unprecedented in clinical approaches to protein degradation," Bradner said in a statement
Their conjugated JQ1-phthalimide drug - simplified to the name dBET1 - showed impressive selectivity by degrading only the three intended protein targets of JQ1 (BRD2, 3 and 4) while leaving the remainder of the proteins in the cancerous cell unaltered.
Traditionally, small-molecules targeted against multidomain scaffold proteins only inhibit one domain in their target proteins, and as such, cancerous cells may exploit alternative signaling pathways for the cells survival and growth. This leads to the major setback with cancer treatment which is drug resistance. But what's neat about this technology is that it doesn't block a single aberrant signalling pathway--it permanently destroys and disposes of the entire protein responsible.
So far, dBET1 has shown promising results against leukemia cells in preclinical experiments with "few noticeable side effects". More importantly, BET-family degradation by dBET1 has clear translational value in reducing the size of the tumor, at least in a mouse model of leukemia.
This technology has given the Dana-Farber researchers a platform to test other conjugates, such as dFKBP12 in other cancers, as well as nonmalignant diseases and even rare genetic disorders.
The dBET1 and dFKBP12 compounds are currently in the late stage of lead optimization.
- here's the abstract
- and here's the Dana Farber release