At first glance, you might suspect that Gene Signal's Phase III study of its lead drug for a rare eye disease was a roaring success. But if you dig down into its release, you'll find that the drug flunked its primary endpoint--an inconvenient truth that is dismissed as a mere triviality.
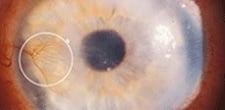 |
| Corneal neovascularization--Courtesy of Gene Signal |
Regulators recommended that Gene Signal use visual acuity as the primary measure of success for aganirsen, which the biotech explains is a common goal for trials of back-of-the-eye drugs. But aganirsen is for a front-of-the-eye problem caused by the abnormal development of blood vessels--corneal neovascularization--that can lead to corneal transplants.
As for visual acuity, Lausanne, Switzerland-based Gene Signal concedes that there was no difference between the drug arm and a placebo. But if you look at the response to corneal neovascularization--CNV--in patient groups suffering from eye inflammation and central neovascularization, the drug "significantly" reduced the need for corneal transplants. It also "tended" to reduce the risk of graft rejection after 90 days of treatment.
Now the biotech says it can whip up a quick new pivotal study to get the data it needs to convince regulators. Provided, of course, that a new trial devoted to a new endpoint can succeed.
"We are confident that these results will enable us to agree with the regulatory authorities a short confirmatory and pivotal study," CEO Eric Viaud said in a statement brimming with confidence. "Our goal is to move quickly to bring a much-needed therapy to the estimated 20,500 corneal neovascularisation graft patients across Europe. Aganirsen is set to become a break-through topical therapy for a range of ophthalmic diseases."
Aganirsen looks to blunt the effects of VEGF, which drives neovascularization in the eye. Viaud also has been touting the drug's potential in taking on some of the giant franchises in eye disease. In an interview with Reuters Viaud said that the company is in talks with potential partners for advancing the therapy as a treatment for wet, age-related macular degeneration, a common affliction of the elderly now treated by Eylea and Lucentis.
There's nothing unusual about partnership talks at a biotech company. But the news may help blunt a Phase III failure, which the biotech is anxious to explain away.
- here's the release (PDF)
- read the Reuters report