 |
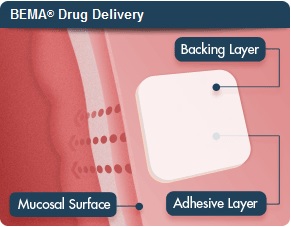
| BioDelivery Sciences' lead drug is a dissolving oral film.--Courtesy of BioDelivery Sciences |
Raleigh, NC's BioDelivery Sciences ($BDSI) is expecting to win FDA approval for its opioid-dependence patch this summer, and the biotech has enlisted global contractor Quintiles ($Q) to handle the launch.
The drug, Bunavail, is a dissolving film placed on the inner lining of the cheek, using buprenorphine to help wean patients off of opioids. The FDA is set to make a final ruling on it by June 7, and, assuming the oral patch wins out, BioDelivery expects to launch Bunavail toward the end of the third quarter, and that's where Quintiles and its commercial heft comes in.
The CRO has agreed to do the heavy lifting in launching the drug, recruiting, training and deploying a sales force to target the roughly 5,000 physicians who account for 90% of buprenorphine prescriptions for opioid dependence, according to BioDelivery.
The drug developer raised about $60 million last month to fund Bunavail's planned launch, using the cash to sign separate deals with Quintiles and contractor Ashfield Market Access, which will handle payer strategy for the drug.
"Leading this collaborative effort with Quintiles, BDSI will be in a position to build an experienced and highly committed sales force," said David Acheson, BDSI's vice president of sales, in a statement. "Additionally, the added strength of Ashfield Market Access provides us critical access to trade and managed markets by tapping into the resources of individuals with significant experience and long-term relationships with payers."
Quintiles' service platform spans from drug discovery to market access, and the world's largest CRO has been working to amp up its end-stage work, in January teaming up with the Medical Affairs Company to expand its capabilities in commercialization.
- read the announcement