Though mammograms are seen as the current “gold standard” for breast cancer diagnosis, ultrasounds can actually provide a more accurate image of the breast tissue in younger women and others with denser tissue in addition to offering a less expensive, radiation-free alternative to mammography.
Now, thanks to San Francisco-based iSono Health—with an assist from the FDA—whole-breast ultrasounds are even easier and faster to perform.
The startup announced Tuesday that the regulator has approved its Atusa technology, a portable ultrasound system that uses machine learning artificial intelligence to generate whole-breast images within just a few minutes.
The wearable Atusa system looks something like the high-tech inverse of Madonna’s infamous cone bra. Once a patient is strapped in and laying on an examination table, their clinician activates the system with a push of a button; the system doesn’t require a specially trained ultrasound technologist to operate.
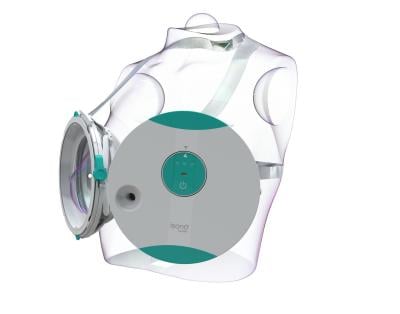
Scanning the entire breast volume takes only about one minute per side—a far cry from the 10 to 15 minutes it can take to complete manual probing.
After the scans are complete, iSono’s technology immediately provides 2D images of the breast tissue. Meanwhile, its AI begins generating 3D images that physicians can access on a computer or tablet, where they can interact with the images and access radial, coronal, sagittal and transverse views of the tissue to help home in on lesions.
Next up, iSono said it’s already beginning to study new deep learning AI models that could be integrated into the Atusa system to automatically localize and classify breast lesions spotted in the system’s collected ultrasound images.
The system was designed to make accurate breast cancer diagnoses more accessible to women around the world; the disease is the leading cause of cancer death.
“We founded iSono Health with the vision to enable earlier diagnosis and treatment for breast cancer to save women’s lives, and this FDA clearance is a major step to fulfilling that vision,” said Maryam Ziaei, Ph.D., iSono’s co-founder and CEO.
“Clinicians and women worldwide need high-quality breast imaging that is accessible and efficient at scale without the need for highly skilled operators,” Ziaei said. “The portable and automated Atusa system stands alone in comparison to other ultrasound offerings in promising to address that need.”