Another crop of health-focused additions to Apple’s eponymous wearable device is ready for harvest. The tech giant unveiled the new features during a keynote presentation Wednesday, with the rollout slated to begin later this month.
The additions to the Apple Watch’s already broad suite of health-tracking features come in the form of one significant hardware update—embedded into two of the three new models of the device that debuted at the keynote—and a handful of software features that build on the device’s existing tech specs, many of which will be downloadable to previous models of the wearable.
First, the new hardware. After years of speculation, Apple has finally added body temperature measurements to the device, catching up to competitor Fitbit, which added the ability to track changes in skin temperature to its own wearables in 2020.
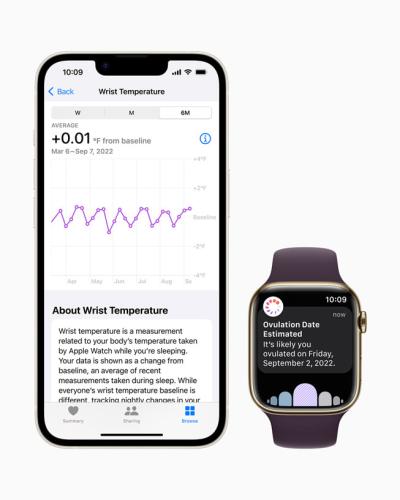
The new Series 8 and Ultra models of the Apple Watch will incorporate two new sensors: one on the back of the device, to measure the body’s temperature through the wrist, and one under the watch’s display, to factor outside temperature into the user’s measurements.
When worn overnight, the wearable will gather temperature measurements every five seconds, according to a company news release, allowing users to monitor fluctuations in their baseline body temperatures as small as 1 degree Celsius that may be caused by illness, exercise, jet lag and more.
Beyond that basic temperature monitoring, Apple is specifically targeting menstrual health with the new feature. The temperature readings will be used to improve the period predictions for those using the Cycle Tracking feature of Apple’s Health app. It will also retroactively estimate when ovulation occurred, potentially helping with family planning, according to the devicemaker.
In addition, Apple’s upcoming iOS 16 and watchOS 9 software updates to the iPhone and Apple Watch, respectively, introduces another new Cycle Tracking feature that will alert users to possible deviations in their menstrual cycles that may be linked to an underlying health condition.
Among the other software updates to the Apple Watch’s health features is the official debut of “AFib History,” which received FDA clearance at the beginning of June. Available to all watchOS 9 users, the technology is aimed at those aged 22 and older who have been diagnosed with atrial fibrillation.
The feature uses the device’s built-in ECG and irregular rhythm notifications—which were previously cleared by the FDA—to compile weekly reports of users’ heart health, indicating how often they showed signs of an irregular or rapid heartbeat. The summaries are presented alongside sleep, alcohol consumption, exercise and other measurements to show how lifestyle factors may be impacting a user’s afib diagnosis.
The updated operating systems will also offer a new Medications app that’ll send pre-programmed reminders and can be used to track medication adherence, as well as a new sleep-tracking feature that can measure not only how heart and respiratory rate change throughout the night but also estimates how much time was spent in each stage of sleep.