Augmenix has snagged $6 million from existing investors in a Series E round. This funding will give a boost to next-gen products and will keep the company’s upward momentum--thanks to the commercial uptake of SpaceOAR--going.
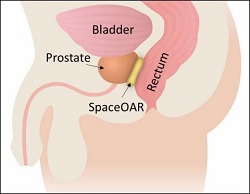
SpaceOAR is an injectable hydrogel that is administered to a patient via a needle. The hydrogel pushes the rectum away from the prostate during prostate cancer radiotherapy, reducing the risk of radiation injury on the rectum.
After being injected in a liquid state, the hydrogel expands and solidifies. It remains in the space between the rectum and prostate for three months during treatment. After that time, the hydrogel is absorbed and cleared out of the body via the patient’s urine.
This results in fewer long-term complications after radiation and improves the patient’s quality of life, Augmenix said.
“With this financing we are thrilled to accelerate the expansion of commercial operations in the U.S. and internationally and we intend to develop devices to space other organs at risk,” said John Pedersen, Augmenix CEO.
They hydrogel tech was cleared by the FDA in April 2015 and is currently being used by more than 129 cancer centers across 35 states. This funding could help develop uses for hydrogel beyond those relating to prostate cancer, Augmenix’s President Pat Campbell explained.
“There was great interest in hydrogel technology at the recent World Conference of Interventional Oncology where hydrogel spacers could protect healthy tissues during interventional procedures,” Campbell said.
SpaceOAR also has a CE mark and Canadian and TGA approvals.
- here's the press release
Related Articles:
Augmenix aims for profitability with $10.8M Series D round as Varian acquisition option expires
Augmenix unleashes promising data for injectable prostate cancer spacer