Novartis ($NVS) says that a pivotal study of its long-acting version of Signifor demonstrated success in treating acromegaly, a condition in which excess levels of growth hormone causes body parts like the hands and feet and face to swell.
At the meeting of the European Congress of Endocrinology, investigators said that patients taking pasireotide long-acting release "achieved greater disease control when compared to continued treatment with the standard somatostatin analogue therapies, octreotide LAR or lanreotide Autogel."
 |
| Dr. Monica Gadelha |
"Historically, we have evaluated somatostatin analogues for the treatment of acromegaly by the decrease in either growth hormone or insulin-like growth factor levels. With more sensitive assays and more stringent evaluation criteria, a recent meta-analysis indicates that up to 45% of patients can have either GH or IGF-I still elevated," said Dr. Monica Gadelha, a professor at the Federal University of Rio de Janeiro and the primary study author. "As the health risks associated with acromegaly may persist until both GH and IGF-1 levels are normalized, this study further supports the importance of monitoring for and achieving full biochemical control."
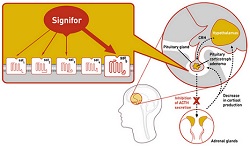 |
| Signifor's mechanism of action in the brain--Courtesy of Novartis |
This new data tied to earlier results are being used to back new regulatory filings for long-acting Signifor.
"These results strengthen our understanding of this rare endocrine disorder and suggest pasireotide LAR may offer benefit for acromegaly patients whose disease is not fully controlled on their current therapy," said Alessandro Riva, the global head of Oncology Development and Medical Affairs. "As part of our long-standing commitment to transforming the care of rare pituitary diseases, we are working to bring this potentially meaningful solution to the acromegaly community."
- here's the release