 |
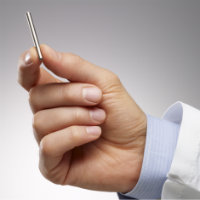
| Intarcia's ITCA 650 is a subcutaneous implant that administers exenatide.--Courtesy of Intarcia |
Well-funded biotech Intarcia Therapeutics is rolling along with its ambitious plans to develop a diabetes treatment without Big Pharma's help, posting positive results from two late-stage studies on its once-a-year drug-device combo.
Intarcia's product, ITCA 650, is an under-the-skin minipump armed with the GLP-1 receptor agonist exenatide--also known as AstraZeneca's ($AZN) Bydureon--for up to one year.
In its first Phase III trial, Intarcia enrolled 460 patients with Type 2 diabetes and blood glucose levels between 7.5% and 10%. After 39 weeks, ITCA 650 met its primary endpoint of significantly beating out placebo in reducing blood sugar and cleared secondary goals of weight loss and percentage of patients who reached their goal glucose levels of under 7%. In a second, open-label study on patients with blood glucose levels over 10%, ITCA got 25% of participants down below the 7% threshold and charted a sustained 3.4% reduction at 9 months.
Intarcia is calling that a major victory. Current GLP-1 therapies, led by Novo Nordisk's ($NVO) blockbuster Victoza, require daily or weekly injections to get diabetes down to their target blood sugar concentrations. If ITCA 650 can match its rivals on efficacy, its promise of a once-a-year procedure could disrupt the still-growing GLP-1 market.
The new clinical data spell a huge milestone for Intarcia, which closed a $200 million funding round in April and has steadfastly refused to seek a partner for its top prospect. Now, according to CEO Kurt Graves, that commitment is looking prudent.
"Many thought an emerging biotech company could never innovate enough, finance enough or execute well enough to bring a transformational therapy through Phase III in a major disease area like Type 2 diabetes," Graves said in a statement. "But we've proven it can be done, and done well."
But the company still has plenty of work to do. Intarcia is in the midst of a third Phase III trial, in which it has enrolled 500 patients to compare ITCA 650 to Merck's ($MRK) top-selling Januvia, a drug that lowers blood glucose by blocking a protein called DPP-4. That study is on track to wrap up in the summer, and, if all goes according to plan, Intarcia expects to submit its product for FDA review in 2016.
- read the results
Special Reports: What's next in diabetes drugs? The pulse of R&D at ADA14 | FierceBiotech's 2007 Fierce 15 - Intarcia Therapeutics